VIVINEX™ - iSert®
| Vivinex™ iSert® |
| Model name | XC1 | XY1 | ||||
| Optic design | Aspheric design with square, thin and textured optic edge | ||||
| Optic & haptic materials | Hydrophobic acrylic Vivinex™ with UV-filter (Model XC1), with UV- and blue light filter (Model XY1) | ||||
| Haptic design | Textured-rough haptic surface | ||||
| Diameter (optic/OAL) | 6.00 mm/13.00 mm | ||||
| Power | +6.00 to +30.00 D (in 0.50 D increments) | ||||
| Nominal A-constant* | 118.9 | ||||
| Optimized constants† | Haigis | a0 = -0.8028 | a1 = 0.2133 | a2 = 0.2245 | |
| Hoffer Q | pACD = 5.697 | ||||
| Holladay 1 | sf = 1.934 | ||||
| SRK/T | A = 119.198 | ||||
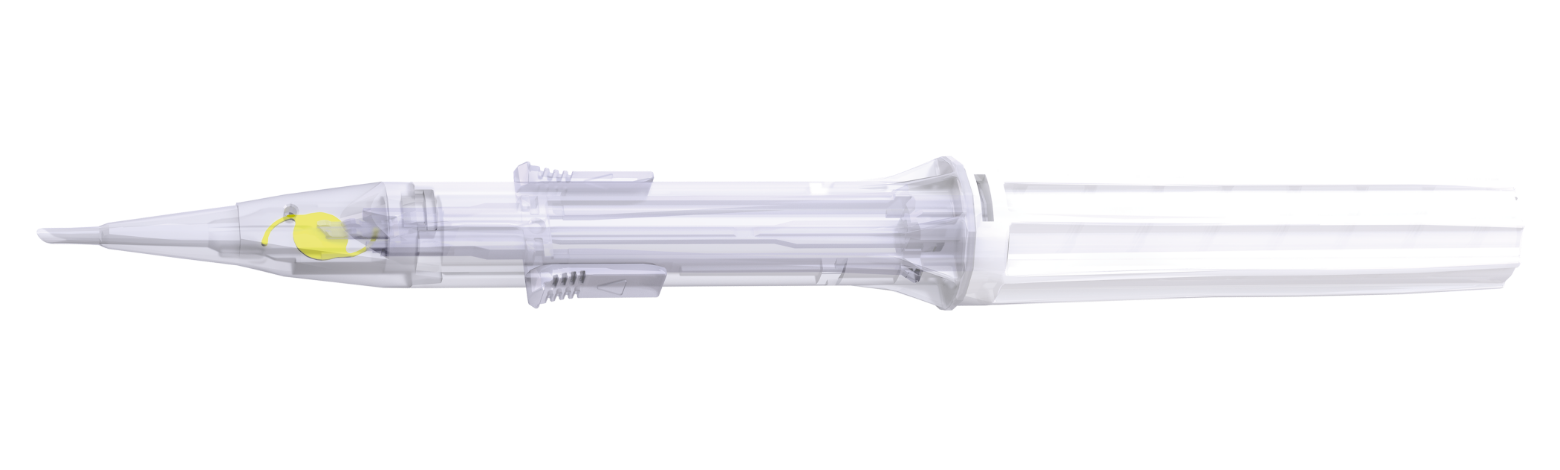
| Injector | Vivinex™ iSert® preloaded | ||||
| Front injector tip outer diameter | 1.70 mm | ||||
| Recommended incision size | 2.20 mm | ||||
*The A-constant is presented as a starting point for the lens power calculation. When calculating the exact lens power, it is recommended that calculations be performed individually, based on the equipment used and operating surgeon’s own experience.
†These optimized constants for the calculation of intraocular lens power published by IOLCon on their website: https://iolcon.org are calculated from 1,475 clinical results for Vivinex™ model XY1/XC1 as of September 24, 2021. These constants are based on actual surgical data and are provided by IOLCon as a starting point for individual constant optimizations. The information available on the website is based on data originating from other users and not by HOYA Surgical Optics (“HSO”). HSO therefore does not warrant the correctness, completeness and currentness of the contents on the said website.
Information contained is intended for health care professionals unless specifically designed for use with patients. For a full list of indications and contraindications please refer to the Instructions For Use. Some of the products and/or specific features as well as the procedures featured in this document may not be approved in your country and thus may not be available there. Design and specifications are subject to change without prior notice as a result of ongoing technical development. Please contact our regional representative regarding individual availability in your country. HOYA, Vivinex and iSert are trademarks of the HOYA Corporation or its affiliates. ©2024 HOYA Medical Singapore Pte. Ltd. All rights reserved.
Experience smooth surgery with Vivinex™ iSert®
1. Galor, A. er al. (2013): Management strategies to reduce risk of postoperative infections. In Current ophthalmology reports, 1 (4), 10.1007/s40135-013-0021-5.
2. Bodnar, Z. et al. (2012): Toxic anterior segment syndrome: Update on the most common causes. In: Journal of cataract and refractive surgery 38 (11), p. 1902–1910.
3. Jones, J. et al. (2016): The impact of a preloaded intraocular lens delivery system on operating room efficiency in routine cataract surgery. In: Clinical ophthalmology (Auckland, N.Z.), 10, p. 1123–1129.
4. Park, C. et al. (2018): Toxic anterior segment syndrome-an updated review. In: BMC ophthalmology, 18 (1), 276.
5. Chung, B. et al. (2018): Preloaded and non-preloaded intraocular lens delivery system and characteristics: human and porcine eyes trial. In: International journal of ophthalmology, 11 (1), 6–11.
6. Schmidbauer, J. et al. (2002): Rates and causes of intraoperative removal of foldable and rigid intraocular lenses: clinicopathological analysis of 100 cases. In: Journal of cataract and refractive surgery 28 (7), p. 1223–1228.
Consistent and predictable IOL delivery with Vivinex™ iSert®

Scroll