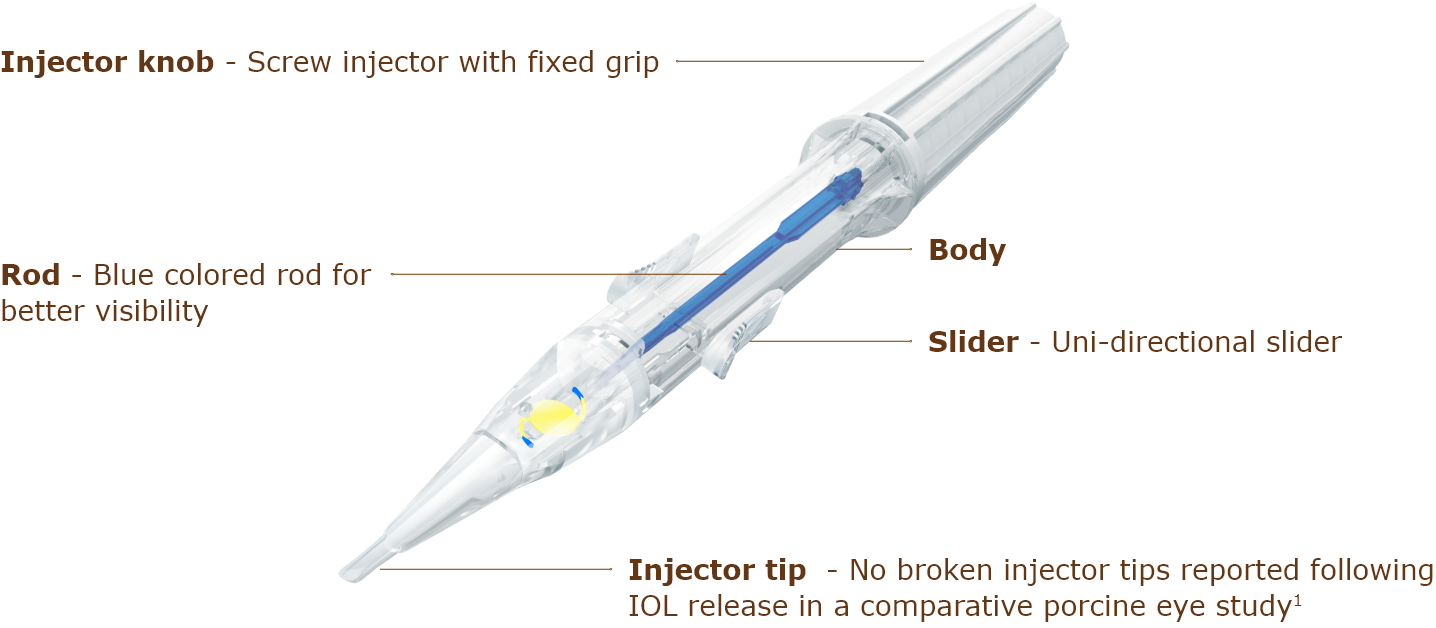
ezsert Injector
| ezSert™ |
| Model name | ezSert™ 255-E* | ezSert™ 254-E* | ||
| Optic design | Aspheric lens design, aberration correcting | |||
| Specification | UV and blue light filter | UV filter | ||
| Optic material | Hydrophobic acrylic (AF-1) | |||
| Manufacturing | Lathe-cut and pad polished | |||
| Haptic material | Hydrophobic acrylic with blue PMMA chemically bonded haptic tips | |||
| Haptic configuration | Modified C-loop, 5° angulation | |||
| Diameter (Optic/OAL) | 6.0 mm/12.5 mm | |||
| Power | +6.00 to +30.00 D (in 0.50 D increment) | |||
| Nominal A-constant† | 118.4 | |||
| Optimized constants‡ | Haigis | a0 = -0.542 | a1 = 0.161 | a2 = 0.204 |
| Hoffer Q | pACD = 5.30 | |||
| Holladay 1 | SF = 1.52 | |||
| SRK/T | A = 118.5 | |||
| Front injector tip outer diameter | 1.78 mm | |||
| Injector | ezSert™ preloaded | |||
*IOLs are the same as iSert® Model 255 and iSert® Model 254.
†The A-constant mentioned above is presented as a guideline only for lens power calculations. It is recommended that the A-constant measurement be customized based on the surgeon‘s experience and measuring equipment.
‡These optimized constants for the calculation of intraocular lens power published by ULIB on their website: http://ocusoft.de/ulib/c1.htm are calculated from 319 clinical results for model 250/251 as of October 31, 2016. These constants are based on actual surgical data and are provided by ULIB as a starting point for individual constant optimizations. The information available on the website is based on data originating from other users and not by HOYA Surgical Optics (HSO). HSO therefore does not warrant the correctness, completeness and currentness of the contents on the said website.
Information contained is intended for health care professionals. For a full list of indications and contra indications please refer to the Instructions For Use. Some of the products and/or specific features as well as the procedures featured in this document may not be approved in your country and thus may not be available there. Please contact our regional representative regarding individual availability in your country. HOYA and ezSert are trademarks of the HOYA Corporation or its affiliates. ©2023 HOYA Medical Singapore Pte. Ltd. All rights reserved
- Comparative porcine eye study: study result (ezSert model 255-E vs manually loaded IOL delivery systems). David J Apple International Laboratory for Ocular Pathology. University Hospital Heidelberg. Report on file.
Experience a new level of surgical ease
*Third-party trademarks used herein are the property of their respective owners.
- Comparative porcine eye study: study result (ezSert model 255-E vs manually loaded IOL delivery systems). David J Apple International Laboratory for Ocular Pathology. University Hospital Heidelberg. Report on file.
Evaluations included a total of 8 procedures and were graded using the seven-point Likert scale:
ease of preparation (IOL loading for cartridge, OVD injection, advancing into nozzle) and ease of implantation (injector body rotation, nozzle insertion, visibility of IOL haptics and rod in the eye, haptics configuration, IOL delivery).
[A] ezSert™ 255-E, +20.0D
[B] AcrySof® IQ SN60WF, +20.0D, MONARCH III, D cartridge
[C] TECNIS® 1-Piece ZCB00, +20.0D, UNFOLDER Platinum 1 Series Implantation System, model 1MTEC30 cartridge
Are you looking for seamless control?
ezSert™ – simpler and faster than manually loaded IOL delivery systems
In a comparative porcine cadaver eye study, ezSert™ achieved:1
- full marks in assessment for “ease of preparation”
- the highest average score in assessment for “ease of implantation”
*Third-party trademarks used herein are the property of their respective owners.
- Comparative porcine eye study: study result (ezSert model 255-E vs manually loaded IOL delivery systems). David J Apple International Laboratory for Ocular Pathology. University Hospital Heidelberg. Report on file.
The priming time for manually loaded IOL delivery systems represents the time needed to load the IOL into the cartridge, inject the OVD and advance the IOL into the nozzle. The priming time for ezSert™ represents the time needed to inject the OVD and advance the IOL into the nozzle (there is no need to load the IOL into the cartridge). The implantation procedure for all three delivery systems represents the time from which the leading haptic exits the nozzle tip, to the time the trailing haptic fully exits the nozzle tip.
[A] ezSertTM 255-E, +20.0D
[B] AcrySof IQ SN60WF, +20.0D, MONARCH III, D cartridge
[C] TECNIS 1-Piece ZCB00, +20.0D, UNFOLDER Platinum 1 Series Implantation System, model 1MTEC30 cartridge
A fast approach for your everyday practice
ezSert™ is significantly faster to use than manually loaded IOL delivery systems1
ezSert™ requires just 15.10s to prime. In comparison, two commonly used manually loaded IOL delivery systems require 73.67s and 54.67s, respectively.
79.5% less priming time versus AcrySof® IQ +MONARCH® III + D cartridge (P < 0.01) 72.4% less priming time versus TECNIS® 1-piece (ZCB00) +UNFOLDER® Platinum 1 Series + 1MTEC30
cartridge (P < 0.01)
Scroll