Nanex IOL
| Nanex™ multiSert+™ | ||||
| Model name | NY1-SP | NC1-SP | ||
|---|---|---|---|---|
| Optic design | Aspheric design with sharp optic edge | |||
| Optic & haptic materials | Hydrophobic acrylic with UV and blue light filter |
Hydrophobic acrylic with UV-Filter |
||
| Haptic design | Modified C-loop, 5° angulation | |||
| Diameter (optic/OAL) |
6.0 mm/13.0 mm | |||
| Power | 6.00 to +30.00 D (in 0.50 D increments) | |||
| Nominal A-constant* |
119.2 | |||
| Injector | multiSert+™ preloaded | |||
| Front injector tip outer diameter |
1.62 mm | |||
| Incision size | As low as 1.8 mm | |||
| Optimized constants† | Haigis | a0 = -0.2676 | a1 = 0.2382 | a2 = 0.1993 |
| Hoffer Q | pACD = 5.715 | |||
| Holladay 1 | SF = 1.904 | |||
| SRK/T | A = 119.112 | |||
*The A-constant is presented as a starting point for the lens power calculation. When calculating the exact lens power, it is recommended that calculations be performed individually, based on the equipment used and operating surgeon’s own experience.
†These optimized constants for the calculation of intraocular lens power published by IOLCon on their website: https://iolcon.org are calculated from 211 clinical results for Nanex™ multiSert+™ model NY1-SP/NC1-SP as of September 9, 2021. These constants are based on actual surgical data and are provided by IOLCon as a starting point for individual constant optimizations. The information available on the website is based on data originating from other users and not by HOYA Surgical Optics (“HSO”). HSO therefore does not warrant the correctness, completeness and currentness of the contents on the said website.
Information contained is intended for health care professionals. For a full list of indications and contra indications please refer to the Instructions For Use. Some of the products and/or specific features as well as the procedures featured in this document may not be approved in your country and thus may not be available there. Please contact our regional representative regarding individual availability in your country. HOYA, Nanex and multiSert+ are trademarks of the HOYA Corporation or its affiliates. ©2023 HOYA Medical Singapore Pte. Ltd. All rights reserved.
- Data on file, HOYA Medical Singapore Pte. Ltd, 2018.
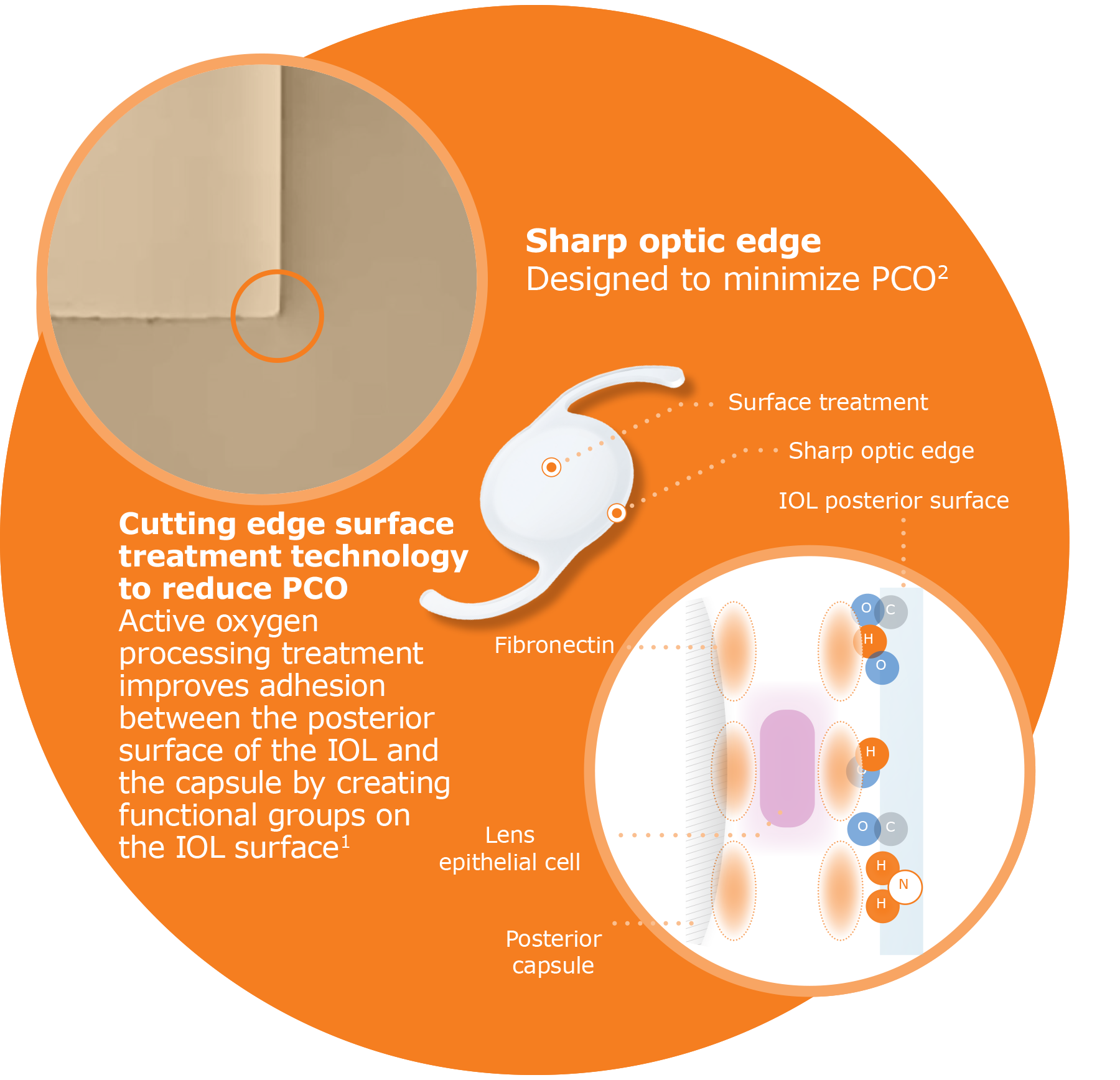
- Matsushima H, et al. Active oxygen processing for acrylic intraocular lenses to prevent posterior capsule opacification. J Cataract Refract Surg. 2006;32:1035-1040.
Our Nanex™ IOLs

Proven hydrophobic acrylic lens material:
over 10 million units implanted worldwide, over 20 years1
Significant PCO reduction:
achieved through HOYA’s proprietary active oxygen processing treatment to the posterior surface of the Nanex™ IOL2
Third-party trademarks used herein are the property of their respective owners.
- Balendiran V, et al. Uveal and capsular biocompatibility of a new hydrophobic acrylic microincision intraocular lens. J Cataract Refract Surg. 2020; 46:459-464.
- Data on file, HOYA Medical Singapore Pte. Ltd, 2019.
Demonstrated PCO reduction
Nanex™ multiSert+™ showed a consistent trend towards stronger PCO inhibition versus AcrySof® IQ across all three scores1,2
In a GLP-study evaluating the PCO of Nanex™ multiSert+™ (NC1-SP) and AcrySof® IQ (SN60WF) in rabbits, while not statistically significant, Nanex™ multiSert+™ showed a trend towards less PCO in comparison with AcrySof® IQ, in the 4-week postoperative study.
- Matsushima H, et al. Active oxygen processing for acrylic intraocular lenses to prevent posterior capsule opacification. J Cataract Refract Surg. 2006; 32:1035-1040.
- Data on file, HOYA Medical Singapore Pte. Ltd, 2019.
What features of an IOL limit PCO?
Scroll